Unique formulation of irinotecan for prolonged circulation
Pancreatic cancer is considered to be a challenge to treat, because the pancreatic tumor is surrounded by a dense wall of tissue. This is known as the stroma, which makes it difficult for some anticancer medicines to reach the tumor.
ONIVYDE was uniquely designed to fight metastatic pancreatic cancer
- A protective shell, called a liposome, surrounds an anticancer drug, called irinotecan
- The liposome helps ONIVYDE stay in circulation in the body, so it can reach the tumor
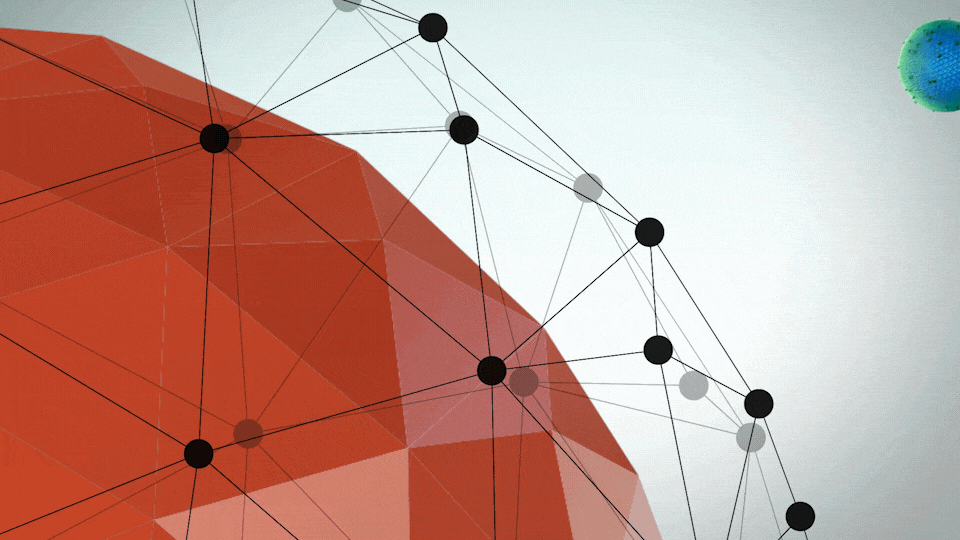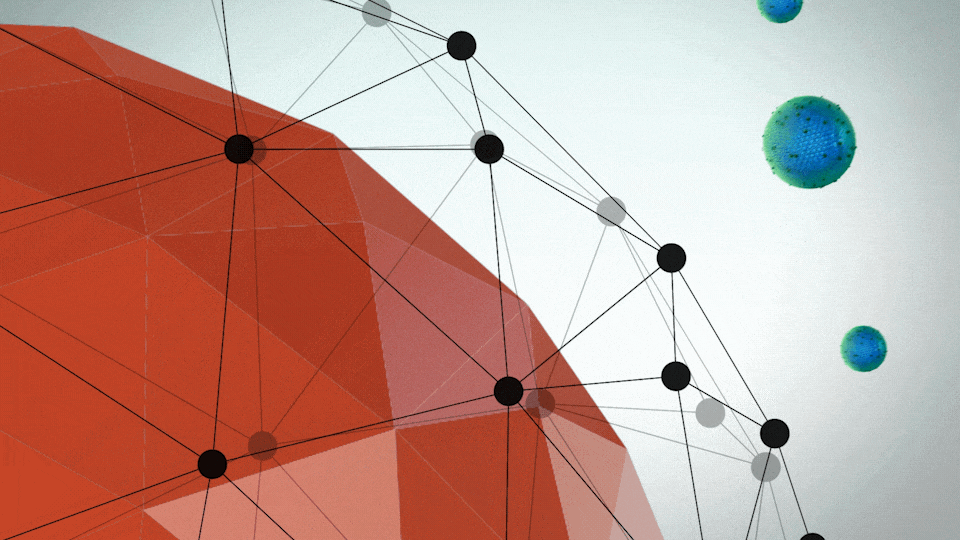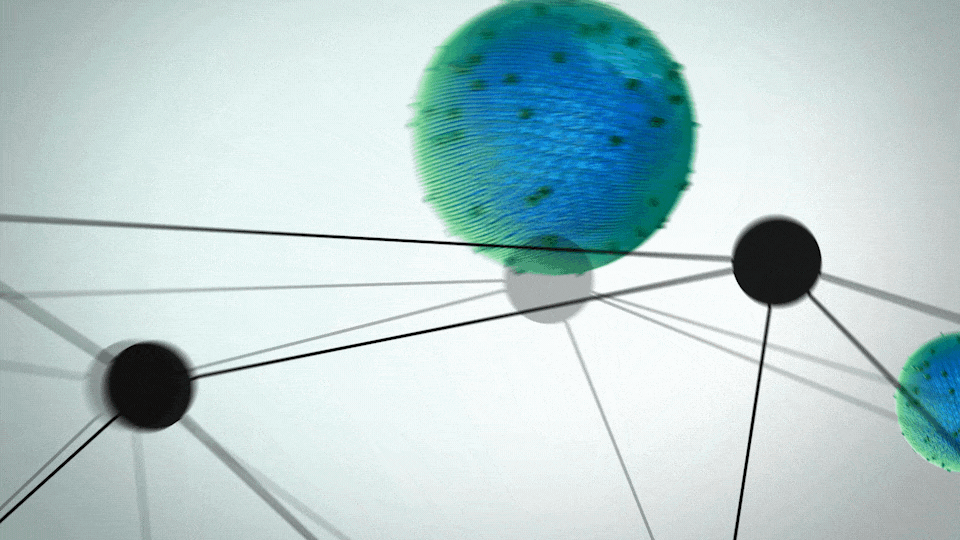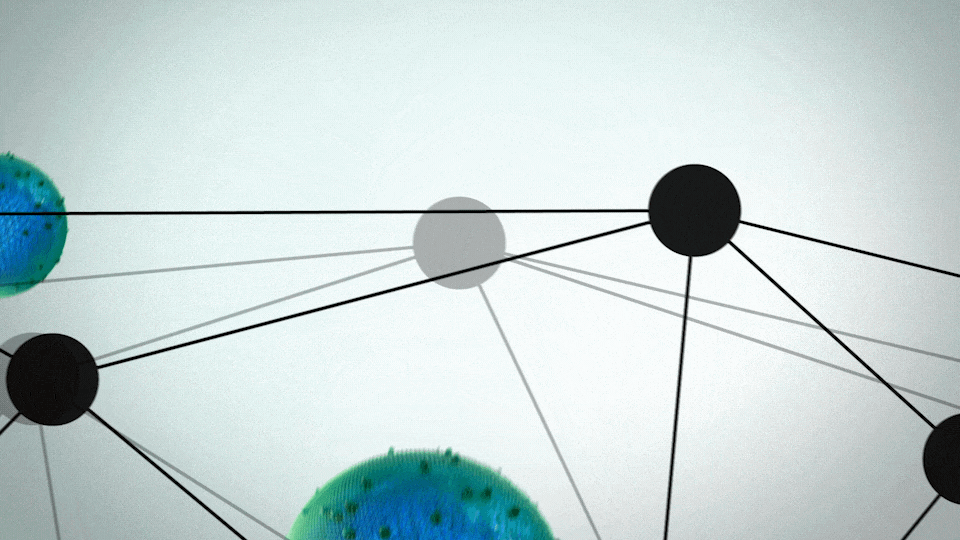
UNIQUE FORMULATION
IMPORTANT SAFETY INFORMATION AND INDICATION
What’s the most important information I need to know about ONIVYDE?
ONIVYDE can cause problems that can sometimes become serious or life threatening and can lead to death. Serious side effects may include fever and infection associated with a low white blood count (neutropenic fever, neutropenic sepsis); diarrhea, lung problems (interstitial lung disease, a group of diseases which cause inflammation of the lung tissues leading to scarring); and reactions during administration of ONIVYDE (including anaphylactic reactions). The most common side effects which were seen in people with pancreatic cancer treated with ONIVYDE include: diarrhea, feeling tired, vomiting, nausea, loss of appetite, inflammation in the mouth, fever, and dehydration. When taking ONIVYDE, you may also have abnormal blood test results. The most common blood count change seen in ONIVYDE-treated pancreatic cancer patients, is a reduction in the number of white blood cells, specifically lower lymphocytes and neutrophils which are important for fighting infections.
Before you receive ONIVYDE, your healthcare provider will give you medications to decrease the potential for allergic reactions to infusion of ONIVYDE. You will also receive anti-nausea medicine to decrease nausea and vomiting, and, possibly, a medicine to decrease immediate diarrhea, called an anti-cholinergic.
What is ONIVYDE used for?
ONIVYDE is a prescription medicine used to treat pancreatic cancer which has spread to other parts of the body. ONIVYDE can be used in patients who have already received gemcitabine treatment for their pancreatic cancer. ONIVYDE is given in combination with 2 other medicines, fluorouracil (also known as 5-FU) and leucovorin (which is often abbreviated as LV), and is not given alone.
When should ONIVYDE not be given?
You should not receive ONIVYDE if:
- you have had a severe allergic reaction to ONIVYDE or irinotecan HCl,
- your white blood cell count is low (neutrophil white blood cell count below the level of 1,500 cells/mm3),
- you have a fever and your neutrophil white blood cell count is low (also called neutropenic fever), or
- you have a problem in your bowel that prevents food, fluids or gas from moving through your intestines.
Serious side effects may occur while taking ONIVYDE. Call or see your healthcare provider right away if you develop any of the following or if these get worse.
Serious side effects may include:
- Infections (particularly if your white blood cells are low). Symptoms of infection may include fever, chills, dizziness, or shortness of breath. Blood cell counts will be monitored periodically by your healthcare provider during treatment.
- Diarrhea. Symptoms of severe diarrhea may include persistent diarrhea; discolored stools (black, green or bloody); or symptoms of dehydration such as lightheadedness, dizziness, or faintness. Your healthcare provider may treat diarrhea with anti-diarrhea medicines (loperamide or atropine).
- Lung problems (interstitial lung disease). Symptoms of interstitial lung disease include new onset of cough or diffculty breathing and fever.
- Allergic reaction (hypersensitivity). Seek immediate medical attention for signs of severe reaction such as chest tightness; shortness of breath; wheezing; dizziness or faintness; or swelling of the face, eyelids, or lips when receiving or during the 24 hours after receiving ONIVYDE.
Getting medical treatment right away may keep these problems from becoming more serious.
Your healthcare provider will check you for these problems during treatment with ONIVYDE. Your healthcare provider may also need to delay or completely stop treatment with ONIVYDE, if you have severe side effects.
- The most frequent side effects resulting in discontinuation of ONIVYDE were diarrhea, vomiting, and infection caused by low white blood cells (neutropenic sepsis).
- The most frequent side effects requiring dose reductions of ONIVYDE were neutropenia, diarrhea, nausea, and low red blood cell count (anemia).
- The most frequent side effects requiring dose interruptions or delays of ONIVYDE were neutropenia, diarrhea, fatigue, vomiting, and low platelet counts called thrombocytopenia (platelets are important for clotting to stop bleeding).
Tell your healthcare provider about all the medicines you take, including: prescriptions, over-the-counter medicines, vitamins and herbal supplements.
Pregnancy and Nursing
If you are a female, tell your healthcare provider if you are pregnant or plan to become pregnant. ONIVYDE can harm your unborn baby. Females who are able to become pregnant should use an effective method of birth control during and for at least 1 month after the last dose of ONIVYDE. Talk to your healthcare provider about birth control methods that you can use during this time. Tell your healthcare provider right away if you become pregnant during treatment with ONIVYDE. Before receiving ONIVYDE, tell your healthcare provider if you are breastfeeding or plan to breastfeed. It is not known if ONIVYDE passes into your breast milk. Do not breastfeed during treatment with ONIVYDE and for at least 1 month after the last dose of ONIVYDE.
If you are a man, you should not father a child during your treatment with ONIVYDE. ONIVYDE can harm the unborn baby of your partner. You should use an effective method of birth control during and for at least 4 months after the last dose of ONIVYDE.
These are not all the possible side effects of ONIVYDE.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. To learn more, talk to your healthcare provider. You can ask your doctor or pharmacist for information about ONIVYDE that is written for health professionals, and it can be found at ONIVYDE.com.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit fda.gov/medwatch or call 1-800-FDA-1088.
Please see full Prescribing Information, including Boxed WARNING.
